BBC World Service
 BBC
BBCThe Indian authorities have prohibited two extremely approaching opioids in response to an investigation by the BBC that found that she feeds a public well being disaster in some elements of West Africa.
In a letter seen by the BBC of the General of the Drug Controller of India, dr. Rajeev Singh Raghuvanshi mentioned that the permission to fabricate and export the medicine has been withdrawn
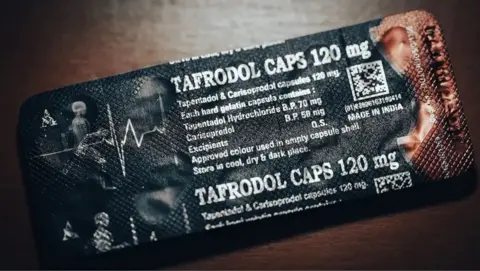
BBC Eye found a pharmaceutical company, Aveo, was illegally exported A blended mixture of Tapentadolo and Carisoprodolo in international locations corresponding to Ghana, Nigeria and Cote D’Ivoire.
The India Food and Drug Administration mentioned that the corporate’s manufacturing unit in Mumbai had been raided and that its complete inventory seized.
The round of dr. Raghuvanshi, dated to Friday, talked about the BBC investigation in his resolution to ban all of the combos of Tapentadolo and Carisoprodolo, which was to be carried out with quick impact.
He mentioned that this additionally got here after officers examined “the potential of drug abuse and its dangerous influence on the inhabitants”.
The tapentadolo is a robust opiate and Carisoprodol is a muscle relaxant, subsequently compelling that’s prohibited in Europe.
Carisoprodolo is accepted to be used within the United States, however just for brief durations of as much as three weeks. Symptoms of abstinence embody nervousness, insomnia and hallucinations.
The mixture of the 2 medicine is just not licensed to be used in any a part of the world as they’ll trigger issue respiratory and convulsions and an overdose can kill.
Despite the dangers, these opioids are fashionable avenue medicine in lots of West African international locations, as a result of they’re so low cost and extensively out there.

The export knowledge out there publicly present that Aveo Pharmaceuticals, along with a subsidiary referred to as Westfin International, despatched hundreds of thousands of those tablets in Ghana and different West African international locations.
The BBC World Service additionally discovered packages of those capsules with the Aveo emblem on sale on the streets of Nigeria and within the cities and cities Ivairian.
Nigeria, with a inhabitants of 225 million individuals, offers the most important marketplace for these capsules. It has been estimated that about 4 million Nigerians abuse some type of opioid, based on the Nation National Bureau of Statistics.
As a part of the investigation, the BBC additionally despatched an secret agent – posing as an African businessman who tried to offer opioids in Nigeria – inside one in every of Aveo’s factories in India, the place they filmed one of many Directors of Aveo, Vinod Sharma, displaying the identical harmful harmful merchandise that the BBC has discovered on sale all through West Africa.
In the secretly recorded film, the operator tells Sharma that his plan is to promote the capsules to youngsters in Nigeria “that everybody loves this product”.
Sharma in response replies “okay”, earlier than explaining that if customers take two or three capsules concurrently, they’ll “calm down” and comply with grow to be “excessive”.
Towards the top of the assembly, Sharma says: “This may be very dangerous to well being”, including that “these days, it’s enterprise”.
Sharma and Aveo Pharmaceuticals didn’t reply to a commentary request when the preliminary investigation of the BBC was printed.
The India Food and Drug Administration mentioned {that a} pungent operation noticed the whole Aveo’s broth seized and the additional manufacturing stopped in a declaration on Friday. Further authorized shares shall be taken in opposition to the corporate, he added.
The company mentioned she was “absolutely ready” to behave in opposition to anybody concerned in “unlawful actions that obscure the fame of the nation”.
The FDA was instructed to carry out additional inspections to stop the provision of medicine, he mentioned.







